Principle Investigator: Roy Price
At the shallow-sea vents off Panarea Island (Italy), hydrothermal venting is manifested as abundant gas and fluid discharge. Vent fluids, pore fluids and gases at three sites were sampled and analyzed for major and minor elements, redox-sensitive compounds, free gas compositions, and strontium isotopes. The corresponding data were used to 1) describe the subsurface geochemical evolution of the fluids and 2) to evaluate the catabolic potential of 61 inorganic redox reactions for in situ microbial communities. Generally, the vent fluids can be hot (up to 135 °C), acidic (pH 1.9-5.7), and sulfidic (up to 2.5 mM H2S). Three distinct types of hydrothermal fluids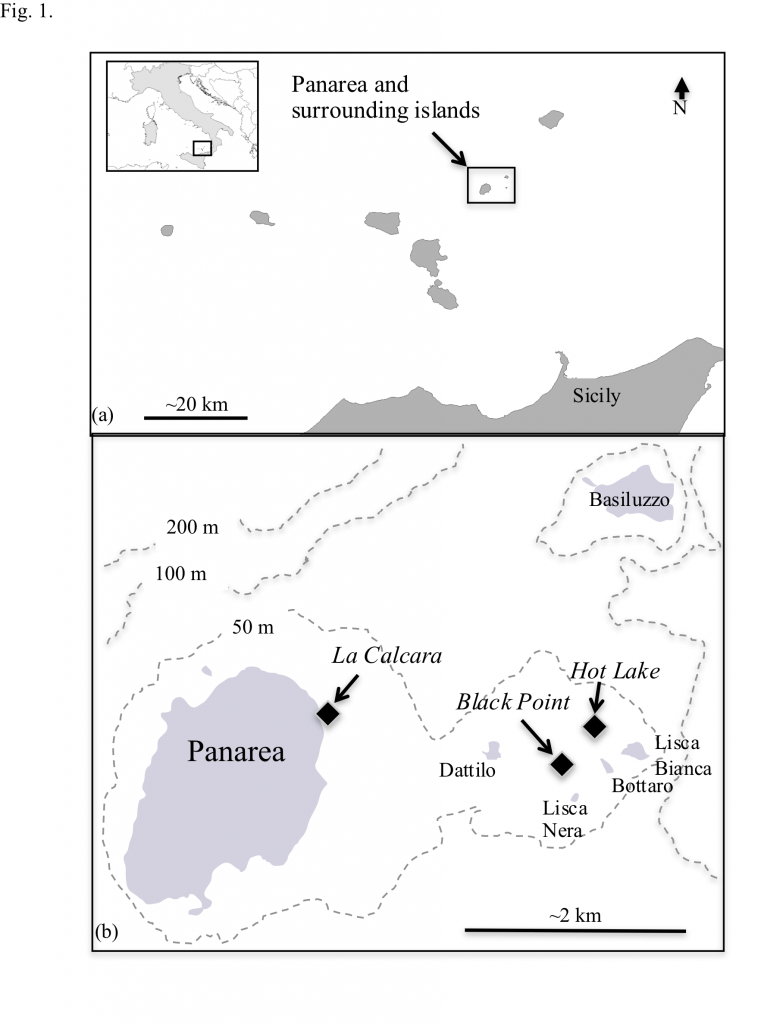 were identified, each with higher temperatures and lower pH, Mg2+ and SO42-, relative to seawater. Type 1 was consistently more saline than Type 2, and both were more saline than seawater. Type 3 fluids were similar to or slightly depleted in most major ions relative to seawater. End-member calculations of conservative elements indicate that Type 1 and Type 2 fluids are derived from two different sources, most likely 1) a deeper, higher salinity reservoir and 2) a shallower, lower salinity reservoir, respectively, in a layered hydrothermal system. The deeper reservoir records some of the highest end-member Cl concentrations to date, and developed as a result of recirculation of brine fluids with long term loss of steam and volatiles due to past phase separation. No strong evidence for ongoing phase separation is observed. Type 3 fluids are suggested to be mostly influenced by degassing of volatiles and subsequently dissolution of CO2, H2S, and other gases into the aqueous phase. Gibbs energies (ΔGr) of redox reactions that couple potential terminal electron acceptors (O2, NO3–, MnIV, FeIII, SO42-, S0, CO2,) with potential electron donors (H2, NH4+, Fe2+, Mn2+, H2S, CH4) were evaluated at in situ temperatures and compositions for each site and by fluid type. When Gibbs energies of reaction are normalized per kilogram of hydrothermal fluid, sulfur oxidation reactions are the most exergonic, while the oxidation of Fe2+, NH4+, CH4, and Mn2+ are moderately energy yielding. The energetics calculations indicate that the most robust microbial communities in the Panarea hot springs combine H2S from deep water-rock-gas interactions with O2 that is entrained via seawater mixing to fuel their activities, regardless of site location or fluid type.
were identified, each with higher temperatures and lower pH, Mg2+ and SO42-, relative to seawater. Type 1 was consistently more saline than Type 2, and both were more saline than seawater. Type 3 fluids were similar to or slightly depleted in most major ions relative to seawater. End-member calculations of conservative elements indicate that Type 1 and Type 2 fluids are derived from two different sources, most likely 1) a deeper, higher salinity reservoir and 2) a shallower, lower salinity reservoir, respectively, in a layered hydrothermal system. The deeper reservoir records some of the highest end-member Cl concentrations to date, and developed as a result of recirculation of brine fluids with long term loss of steam and volatiles due to past phase separation. No strong evidence for ongoing phase separation is observed. Type 3 fluids are suggested to be mostly influenced by degassing of volatiles and subsequently dissolution of CO2, H2S, and other gases into the aqueous phase. Gibbs energies (ΔGr) of redox reactions that couple potential terminal electron acceptors (O2, NO3–, MnIV, FeIII, SO42-, S0, CO2,) with potential electron donors (H2, NH4+, Fe2+, Mn2+, H2S, CH4) were evaluated at in situ temperatures and compositions for each site and by fluid type. When Gibbs energies of reaction are normalized per kilogram of hydrothermal fluid, sulfur oxidation reactions are the most exergonic, while the oxidation of Fe2+, NH4+, CH4, and Mn2+ are moderately energy yielding. The energetics calculations indicate that the most robust microbial communities in the Panarea hot springs combine H2S from deep water-rock-gas interactions with O2 that is entrained via seawater mixing to fuel their activities, regardless of site location or fluid type.