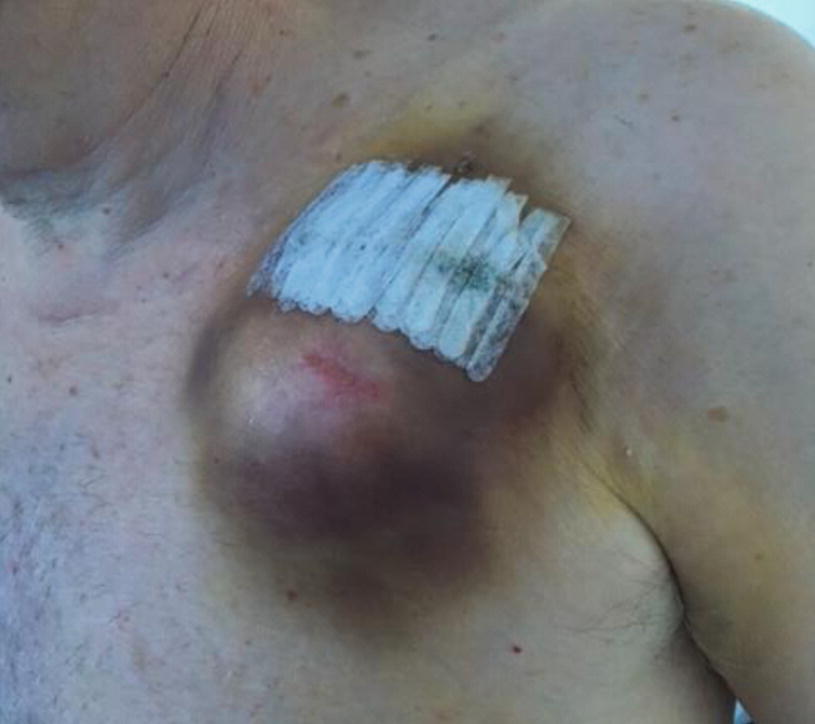
Our goal is to design a device that can decrease hematoma formation at the surgical site at which a pacemaker or defibrillator has been implanted. This is a prevalent issue as approximately 95 out of 1000 patients that receive this procedure develop a hematoma [1].
There are currently few solutions for this which include the use of ice packs and a pressure bandage. As well as this, it was also found that avoiding anticoagulant medication before having the device implanted can also decrease likelihood of a hematoma forming. While these are all effective treatments, they have their limits. This includes skin tears and allergic reactions that occur as a result of the existing pressure bandage.
With this in mind, our group is trying to find a way to prevent hematomas after device implantation with limited complications. To do this, we have made a list of the most important design criteria that will be used a guideline as we develop our design. Our design criteria include a non-invasive approach, decreased hospitalization time, making sure the device is comfortable for the patient, and finding a material that is biocompatible for the patient.
 [2]
[2]