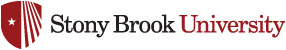CURRENT APPLICATIONS UNDER INVESTIGATION
NARMIL was established in 2014 with a National Science Foundation Major Research Instrumentation Grant through the Division of Ocean Sciences and through matching support from Stony Brook University and has been fully operational for the last year. This facility supports research in marine, atmospheric, environmental, biological, chemical, geological, materials sciences, and biomedical engineering, and is certainly amenable to other applications. The lab provides state-of-the-art instrumentation and expertise for analyses of single cells, aerosols, natural materials, engineered surfaces, minerals, biofilms, thin films, and novel synthetic materials. The lab’s mission is to offer unique analytical solutions to chronic limitations experienced in many research areas, to enable transformative discoveries, and to educate the next generation of scientists. If you have a potential application or questions, don’t hesitate to contact us at NARMIL@stonybrook.edu.
Ms. Tanya Zaliznyak joined the SoMAS staff and took over the helm at NARMIL in September 2015. Her first year has been filled with exciting accomplishments demonstrating that this facility can address a myriad of applications in basic and applied sciences as well as engineering and that NARMIL is realizing its scientific potential. We’d like to share some of these achievements with you.
1) Developed protocols to interrogate single microbial cells and marine aerosol particles in the confocal Raman microspectrometer.
2) Produced sub-micrometer scale Raman maps of cells and engineered materials on the Atomic Force Microscope (AFM) stage by co-locating the Raman laser beam on the AFM tip.
3) Developed software scripts, pipelines and shortcuts to optimize routine Raman spectral analyses.
4) With Prof. Taylor’s group (SoMAS), refined techniques to map distributions of intracellular storage products, such as globular sulfur and polyhydroxybutyrate (Fig. 1).
Figure 1. Raman map of intracellular distributions of the lipid-like energy storage product, polyhydroxybutyrate (red area with accompanying Raman spectrum) within a marine bacterium (below arrow in right panel). Blue area is relatively rich in amino acids (blue spectrum) and proteins. Cell is approximately 2.5 micrometers long.
5) For ongoing studies in Prof. Taylor’s lab (SoMAS), NARMIL improved capabilities for routine Raman-FISH single cell analyses. Technique enables recognition of photosynthetically-active phytoplankton and calculating single-cell growth rates by quantifying stable isotope incorporation into biomolecules by Raman signatures (Fig. 2) and identifying players by genetic probes (FISH = fluorescent in situ hybridization). This work is published in Taylor et al. (2017 )(https://www.frontiersin.org/articles/10.3389/fmicb.2017.01449/full) and summarized in a recently presented poster. Link = Taylor_Gordon_OCB2018_Poster-11rcve1
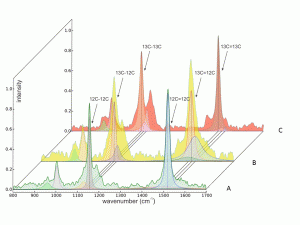
Figure 2. Examples of varying contributions of 12C-12C, 12C-13C, and 13C-13C isotopologues to shape, position, and areas of the n (C-C) and n (C=C) Raman spectral peaks for carotenoids of Synechococcus sp. cells assimilating varying amounts of DI13C. Raman spectra were obtained from individual cells grown in either 1.1% (A) or 50% (B) or 94% 13C-bicarbonate for 9 days. Spectra were baseline corrected, intensity normalized (0-1), and subjected to a full Voigt curve-fitting routine (convolution of Lorentzian and Gaussian profiles) with 5,000 iterations or a 0.00001 tolerance using Renishaw™ Wire 4.1® software.
6) Currently assisting Prof. Taylor’s group (SoMAS) in development of novel Raman and AFM techniques to follow movement of carbon from prey (Fig. 3) through protistan symbiotic associations, between hosts and viral pathogens, and from detritus through microbial communities. This new project is supported by the Gordon & Betty Moore Foundation and funds two new postdoctoral investigators.
Figure 3. Raman spectra from single heterotrophic bacterial cells grown in organic media with either natural 13C abundances (blue spectrum) or with highly 13C –enriched substrates (red spectrum). Peaks experiencing significant “red-shifts” due to heavy isotope enrichment are labeled.
7) Facilitated Profs. Knopf and J. Aller group’s (SoMAS) exploration of chemical variability in sea spray aerosols and their role in cloud formation using Raman spectroscopy (Fig. 4).
Figure 4. Raman spectrum of a single aqueous sea spray droplet (marked by red arrow in panel on right) that contains biogenic polysaccharidic and proteinaceous material obtained from a laboratory mesocosm experiment.
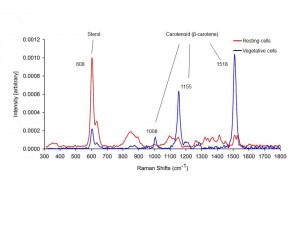
8) Assisted Ms. Yoonja Kang, Prof. Gobler’s student (SoMAS), to spectroscopically characterize how cellular storage products and pigment concentrations change when a harmful bloom-forming microalga (Aureoumbra lagunensis) enters a resting stage (Fig. 5).
Figure 5. Examples of single-cell Raman spectra (left) acquired from dried resting A. lagunensis cells (red) and vegetative (blue) cells under ambient laboratory conditions. Light microscope and TEM images (right). (A) light microscope image of vegetative cells at 21ºC, (B) light microscope image of resting cells formed at 35°C and enlarged image of Aureoumbra resting cell (inset), (C) TEM image of vegetative cell, (D) TEM image of resting cell. Abbreviations are C = cytoplasm, Sd = sterol-enriched droplet, Ga = Golgi apparatus, M = mitochondria, P = plastid, Pg = plastoglobuli, and Tk = thylakoids (Kang et al. 2016 J. Phycol.).
9) Facilitated Prof. Robert Aller’s group (SoMAS) in spectroscopically characterizing effects of animal-sediment interactions on biogeochemical processes near the sediment-water interface.
10) Assisted Ms. Emily Herstoff, Prof. Stephen Baine’s student (Ecology & Evolution), by performing single-cell Raman analyses on an array of cultured microalgae to characterize differences in macromolecular composition.
11) Facilitated Prof. Irena Tannenbaum (Materials Sciences and Engineering) and undergraduates in characterizing interactions between biomolecules and gold nanoparticles using Raman microspectroscopy.
12) Assisted Prof. Balaji Sitharaman’s group (Biomedical Engineering) in study of bioactivity of some novel nanocomposites for bone tissue engineering using Raman microspectroscopy.
13) Provided spectral data to Prof. Yizhi Meng’s group (Materials Sciences and Engineering) to guide establishment of a 3D spheroid model of breast microcalcifcation.
14) With Prof. Stanislaus Wong’s group (Chemistry) expanded Raman spectral characterization of carbon nanotubule functionalization, primarily for photovoltaic applications.
15) Provided spectral data to Prof. Nancy Goroff’s group (Chemistry) to enable their evaluation of methods for preparing carbon-rich and all-carbon materials via self-assembly.