Microorganisms are highly adaptable and are developing novel resistance mechanisms, diminishing the effectiveness of our current therapeutic repertoire. As a result, antimicrobial resistance (AMR) is a growing global health threat. AMR was associated with 4.95 million deaths in 2019 and the mortality rate is expected to grow and cause greater burden on healthcare systems [1]. Thus, novel treatment strategies are needed.

To overcome AMR, one group is straying away from the path of developing small molecule drugs and is instead engineering bacteria as a form of medicine. Researchers in Luis Serrano’s group at the Centre for Genomic Regulation (Barcelona, Spain) modified Mycoplasma pneumoniae to attack Pseudomonas aeruginosa [2]. Their findings are published in Nature Biotechnology in the paper, “Engineered live bacteria suppress Pseudomonas aeruginosa infection in mouse lung and dissolve endotracheal-tube biofilms.”.
Pseudomonas aeruginosa is an opportunistic pathogen that is the cause of many human infections, including pneumonia, osteomyelitis, skin infection, implant-associated infection, UTIs and catheter-associated infection [3]. P. aeruginosa can also survive as biofilms, layers of bacteria that are adherent to tissues and other materials, including medical equipment and devices. These biofilms are especially harmful, as they can form on endotracheal tubes used for patients on ventilators, leading to ventilator associated infections. These infections worsen a patient’s conditions and lead to extended stays in the hospital as well as increased mortality risk.
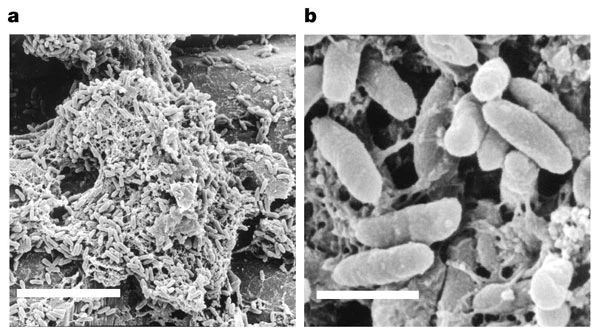
Source: Whiteley, M., et al. , Nature (2001).
Meanwhile, Mycoplasma pneumoniae is a small Gram-negative bacterium that is known to cause mild respiratory infections in humans. These infections are usually self-limiting but can also be overcome with antibiotics as they lack a cell wall. Researchers determined that M. pneumoniae would be an ideal candidate for development as a “live medicine” due to its susceptibility to antibiotics, its small genome, and the wealth of information available on the pathogen that make gene editing a possibility[2]. Therefore, M. pneumoniae could be modified to attack biofilms and be used in conjunction with other antibiotics to tackle P. aeruginosa respiratory infections without collateral inflammation or damage to the host.

Source: Sauteur, P.M.M., et al., Frontiers Microbiology (2016).
First, specific pathogenic genes were eliminated from M. pneumoniae. The resulting strain was able to colonize and persist in the lung similar to the original wildtype strain, however it did not induce expression of inflammatory markers. This suggested that the attenuated bacteria would not exacerbate the host during infection and adequate numbers of bacteria would be available to target biofilms. Next, enzymes and pyocins, toxins produced by Pseudomonas species that are effective at killing P. aeruginosa were introduced into the attenuated strain of M. pneumoniae.
These genes introduced antibiofilm and antimicrobial properties to M. pneumoniae. The strain was able to dissolve biofilms collected from patients in vitro. In a mouse model, it was able to reduce the number of bacteria in the lungs, reduce the size of lesions and induce less inflammation.
The group is continuing to evaluate other beneficial modifications including the addition of anti-inflammatory cytokines to reduce lung inflammation during respiratory infection [4]. This component of their work is published in Molecular Systems Biology in the paper, “Bacterial expression of a designed single-chain IL-10 prevents severe lung inflammation.”
References:
[1] ‘Lancet Report: Antibiotic resistance leading cause of death’. https://www.twn.my/title2/health.info/2022/hi220113.htm (accessed Mar. 28, 2023).
[2] R. Mazzolini et al., ‘Engineered live bacteria suppress Pseudomonas aeruginosa infection in mouse lung and dissolve endotracheal-tube biofilms’, Nature Biotechnology 2023, pp. 1–10, Jan. 2023, doi: 10.1038/s41587-022-01584-9.
[3] F. F. Tuon, L. R. Dantas, P. H. Suss, and V. S. Tasca Ribeiro, ‘Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review’, Pathogens 2022, Vol. 11, Page 300, vol. 11, no. 3, p. 300, Feb. 2022, doi: 10.3390/PATHOGENS11030300.
[4] A. Montero‐Blay et al., ‘Bacterial expression of a designed single‐chain <scp>IL</scp> ‐10 prevents severe lung inflammation’, Mol Syst Biol, vol. 19, no. 1, Jan. 2023, doi: 10.15252/msb.202211037.
