Image above: Raman microspectroscopy helps researchers observe cell chemistry by focusing a laser beam on a spot within a cell and then capturing light emitted after interactions with cellular molecules, which produces a molecular fingerprint (Raman spectrum).
From “Cell Chemistry Illuminated by Laser Light” on Stony Brook News, November 4, 2019.
Published paper shows tearing down the ‘fluorescent curtain’ opens door to better microspectroscopy
STONY BROOK, NY, November 4, 2019 – Raman microspectroscopy is a laboratory technique to produce molecular fingerprints of materials and biological specimens. However, for many years fluorescence has interfered with effective application of this technique and limited its use. Now Prof. Gordon Taylor of the School of Marine and Atmospheric Sciences (SoMAS) at Stony Brook University, and colleagues in the NAno-RAMAN Molecular Imaging Laboratory (NARMIL) have devised a technique that suppresses fluorescence in sample preparation. This new technique may open the door to more efficient and highly resolved investigations of chemical distributions within individual cells. Their findings are published in Scientific Reports.
Characterizing cell-to-cell and intracellular variations in biochemistry is critical to mechanistic understandings in research that covers a broad area, including cancer, human development, cell biology, antibiotics exploration, and environmental biology. Laser-based Raman microspectroscopy is among only a few tools that scientists can use to effectively observe molecular distributions within intact individual cells.
Taylor and his team demonstrate how this technique overcomes analytical challenges presented by biological samples and figuratively “tears down the fluorescent curtain” in them for laser Raman microspectroscopy interrogation. Through this method they can trace cellular assimilation of isotopic tracers, document intracellular biochemical changes, and analyze diverse environmental samples.
“Previously, the samples we investigated were difficult if not impossible to analyze” says Gordon. “Our new technique could prove to be a game changer for many types of cellular research.”
The investigators so far have used the technique to analyze many cellular conditions, such as examining cell-to-cell variations in growth rates of phytoplankton (microalgae), observing viral infections inside phytoplankton cells, tracing movements of nutrients from marine bacteria into microbial predators, and identifying and quantifying microplastic particles in marine plankton samples.
The research is supported by the Gordon and Betty Moore Foundation (MMI Project #5064) and National Science Foundation grants (OIA-1833053 and OCE 1331336724).
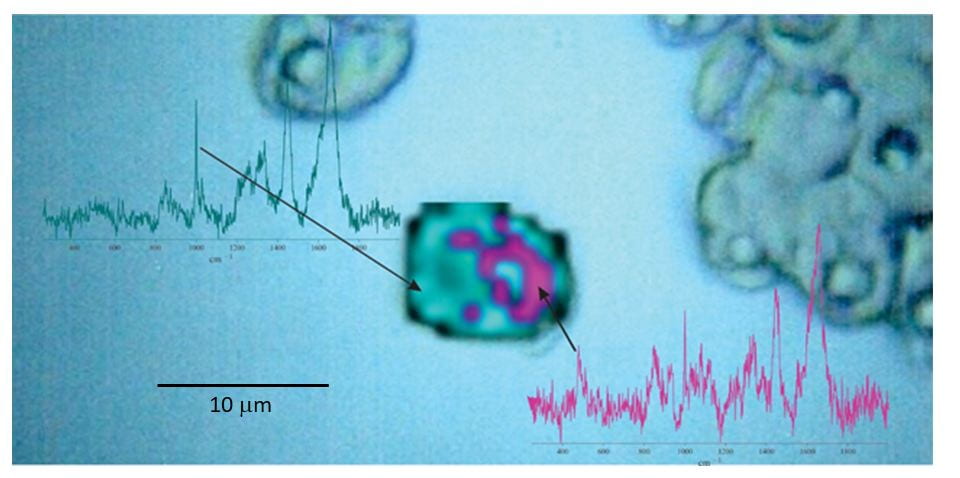
Application of NARMIL’s chemiphotobleaching protocol for Raman microspectroscopic chemical mapping of a formerly highly fluorescent microalgal cell (central image). The map illustrates that starch granules (magenta) surround the cell’s pyrenoid body (blue). The 2-D map is based on spatial distributions and intensities of Raman scattered emissions from starch and protein (blue). Raman spectra from single spots showing diagnostic peaks for starch (478 cm-1) and protein (1001 cm-1) used to produce the 2-D map are presented next to map. Maps and spectra are superimposed on a microscopic image of green algal cells (Tetraselmis levis).
News in Brief
- Characterizing cell-to-cell and intracellular variations in biochemistry is critical to deeper mechanistic understandings in such diverse research areas as cancer, human development, cell biology, antibiotics exploration, and environmental microbiology.
- However, most tools available to researchers are blind to small-scale variations, which substantially limits progress in many lines of biological inquiry.
- Laser-based Raman microspectroscopy is arguably among the few tools that can produce two and three dimensional maps of chemical distributions in cells at sub-micrometer resolution and can fill this information gap.
- However, laser-induced fluorescence has been a serious impediment to probing many biological samples by Raman microspectroscopy.
- Prof. Gordon Taylor’s (SoMAS) research group has devised a technique to suppress nuisance fluorescence during sample preparation that will enable broader application of Raman microspectroscopy to biological studies.
- In this week’s Scientific Reports, Yakubovskaya et al. demonstrate how this technique has overcome analytical challenges presented by an array of fluorescent samples that were previously difficult, if not impossible, to analyze by Raman microspectroscopy.
- Taylor’s group has already used this technology to:
- examine cell-to-cell variations in growth rates of phytoplankton (microalgae)
- trace movement of nutrients from marine bacteria into microbial predators
- observe viral infections inside intact phytoplankton cells
- document production and consumption of energy storage products (fats, starch) in individual phytoplankton cells.
- identify metabolically active microorganisms in complex marine plankton samples
- examine microbial symbioses
- identify and quantify microplastic particles in marine plankton samples
In the future, NARMIL researchers will seek funding to extend these largely lab studies to a variety of marine ecosystems and they anticipate that colleagues will propose many other unexpected applications now that the SoMAS researchers have drawn back the “Fluorescent Curtain”
###
About Stony Brook University
Stony Brook University is going beyond the expectations of what today’s public universities can accomplish. Since its founding in 1957, this young university has grown to become a flagship as one of only four University Center campuses in the State University of New York (SUNY) system with more than 26,000 students and 2,600 faculty members, and 18 NCAA Division I athletic programs. Our faculty have earned numerous prestigious awards, including the Nobel Prize, Pulitzer Prize, Indianapolis Prize for animal conservation, Abel Prize and the inaugural Breakthrough Prize in Mathematics. The University offers students an elite education with an outstanding return on investment: U.S. News & World Report ranks Stony Brook among the top 50 public universities in the nation. Its membership in the Association of American Universities (AAU) places Stony Brook among the top 62 research institutions in North America. As part of the management team of Brookhaven National Laboratory, the University joins a prestigious group of universities that have a role in running federal R&D labs. Stony Brook University is a driving force in the region’s economy, generating nearly 60,000 jobs and an annual economic impact of more than $4.6 billion. Our state, country and world demand ambitious ideas, imaginative solutions and exceptional leadership to forge a better future for all. The students, alumni, researchers and faculty of Stony Brook University are prepared to meet this challenge.
About the School of Marine and Atmospheric Sciences
The School of Marine and Atmospheric Sciences (SoMAS) is a leader in marine, atmospheric and sustainability research; education; public service; and is SUNY’s designated center for the marine sciences. The School is among the leading oceanography and atmospheric sciences institutions in the world, providing students with access to state-of-the-art research laboratories, shipboard experiences, high-powered radar and computing facilities. SoMAS provides expanded study opportunities in the fields of ocean conservation, climate change and extreme weather, sustainability, waste management, marine fisheries and resources, and many others.
Additional Coverage: