Our research focuses on the development of novel, noninvasive in vivo optical imaging techniques.
CURRENT PROJECTS
The Main Research Scheme (Neuro Research & Cancer Research):

Project-1: Time-Gated Diffuse Correlation Spectroscopy for Assessing Human Brain Function (NIH-R01-Brain Initiative, funded)

The diffuse correlation spectroscopy (DCS) technique is an emerging diffuse optical technique for bedside monitoring of blood flow in humans. Currently, DCS operates in continuous-wave (CW) mode, which has limitations such as superficial signal sensitivity and inaccurate quantification of blood flow as a result of the dependence on a priori information of optical parameters. The goal is to address these limitations by proposing a novel technology and method that can quantify both absolute static and dynamic parameters concurrently in a single instrument with fast data acquisition; thus, it is highly suitable for fast functional neuroimaging. An effective way to solve the problem associated with the CW system is to use a time-gating approach (Fig.1). Since single source-detector separation is sufficient for deeper brain sensitivity, this approach can provide simple optical probes useful for neurointensive care unit (neuroICU) settings and potentially high-density fiber optode configuration for improved SNR and spatial resolution. This innovative DCS system and method will result in a quantitative blood flow parameter with enhanced brain sensitivity, thus paving the way for fast clinical translation in neuroICU settings and for general neuroimaging applications.
Publications (related to this project):
Project-2: Non-invasive characterization of Traumatic Brain Injury (TBI) with multi-modal (EEG-Optical) imaging (NIH-R03, 1%, funded)
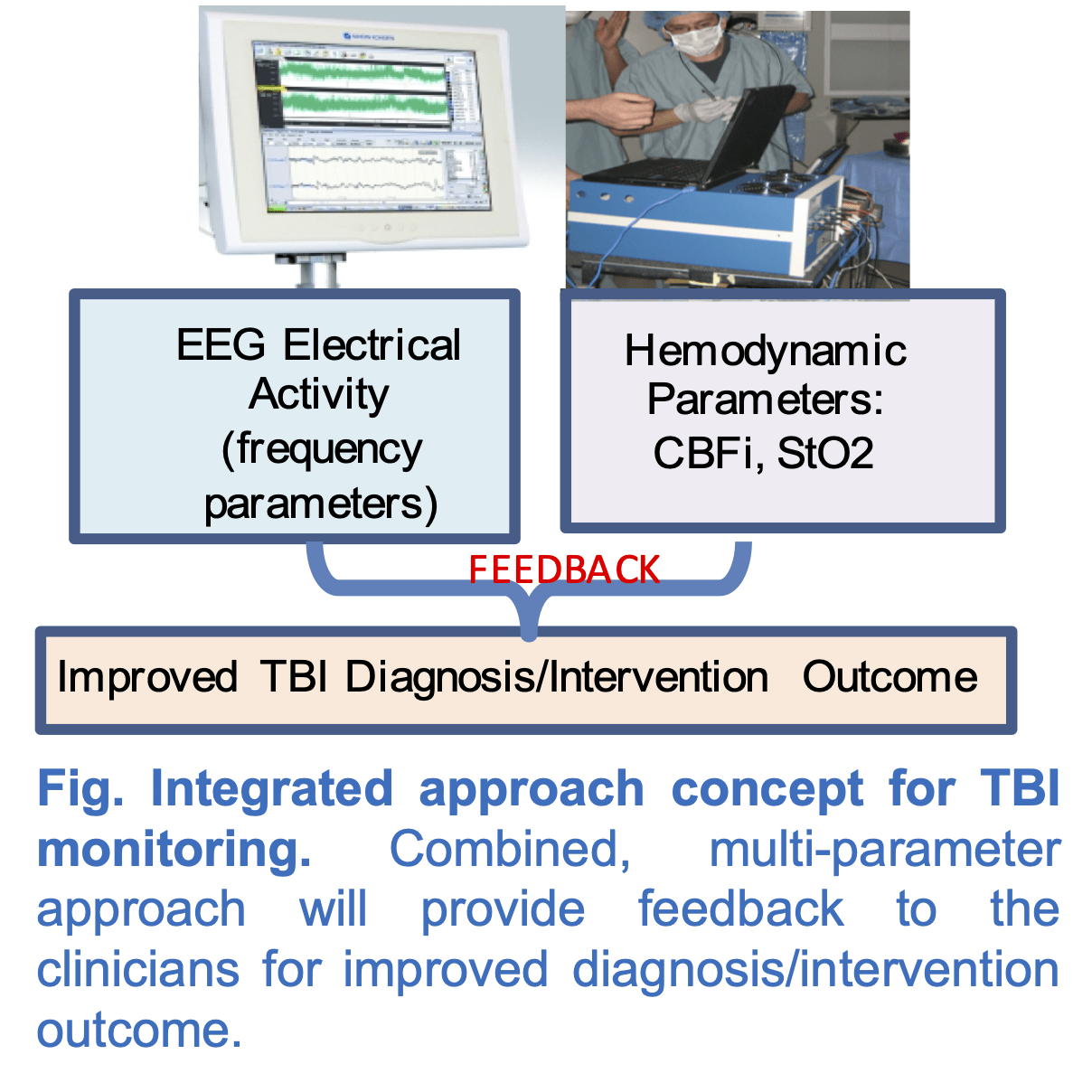
Our goal is to address this need through combined measurements of EEG and functional optical spectroscopy (EEG-Optical) instrumentation and analysis to provide a complementary fusion of data on brain activity and function. EEG records the local electrical field potentials of the brain with exquisite temporal resolution. Optical imaging uses low-intensity light to quantify cerebral blood flow (CBF).
Publications (related to this project):
Project-3:Quantitative Fluorescence Imaging-Guided Detection and Targeted Therapy Monitoring Platform for Ovarian Cancer Metastases (NIH-R01, 4%, funded)

A major challenge in the management of advanced ovarian cancer is the presence of disseminated microscopic metastases (micromets) within the intraperitoneal cavity. We develop a novel approach that can address this challenge by combining treatment planning, light delivery, and tumor response monitoring on a single platform. The approach incorporates quantitative fluorescence imaging for detecting micromets and chemophototherapy (CPT) for destroying them. The approach has the potential to be incorporated into a treatment regime involving keyhole endoscopic surgery for minimally invasive image-guided illumination of metastatic nodules (Fig. 1a). Then, the treatment light intensity and shape can be adjusted according to the absolute fluorescence concentration distribution using the same structured illumination scheme. Figure 1b shows the amenability of spatial control of PoPD-liposomes, which are stable enough to retain their cargo (e.g., doxorubicin) in vivo and release their contents with NIR laser (Fig. 1c). Figure 2 shows the flexible endoscope version of the system. Impact: This project will result in a novel imaging and image-guided therapy monitoring platform that will provide enhanced contrast for detection, as well as complete destruction of ovarian micromets while sparing normal tissue.

Publications (related to this project):
SIDE PROJECTS (to be updated)
» Spatial Frequency Domain Imaging
» Fluorescence Imaging ( » CW ,» Time-domain ):
Fluorescence molecular tomography (FMT) allows noninvasive quantitative imaging of deeply seated tumors with improved contrast. We have developed a time domain FMT system that can quantify quantum yield and lifetime simultaneously. This opens a door for examining photophysical processes and photochemical reactions of fluorescence agents in the local molecular microenvironments (e.g. hypoxia, pH, binding, polarity).
» Spectroscopy (optical blood flow, blood volume and blood oxygenation)
Our recent research area focuses on photoacoustic imaging (PAI). One of the main issues with optical-alone imaging technique is that it suffers from low spatial resolution. To address this issue we like others are combining optics and ultrasound to provide high functional optical contrast at superior ultrasound resolution. Photoacoustic imaging can achieve spatial resolution of a single capillary level (~5µm) at ~1mm, and ~50µm resolution at ~3mm depth. Since it inherits functional optical contrast, it can assess blood vessel vasculature, oxygenation, and flow at high resolution without any contrast agent administration.